On December 18, 2018 it was announced to the media that a study on CBD commercial oils had been carried out in a joint collaboration between Fundación CANNA, the Madrid Salud laboratories and the Observatorio Español de Cannabis Medicinal (OECM).
This study aims to give an overview of the status of these oils in terms of quality. For this purpose, the OECM selected several of these oils based on those that are most used by patients seeking specific health benefits.
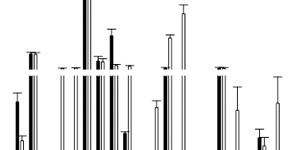
A total of 15 CBD oil products were analysed. Each of these products was purchased at two different times, several months apart, in order to have a quality control of different batches of the same product, so in total 30 samples were analysed.
Both Fundación CANNA and the laboratories of Madrid Salud determined the content of the main phytocannabinoids (CBD and d9-THC) in the oils. Fundación CANNA also quantified the possible acidic forms present (THCa and CBDa) since in some products the CBD content is indicated as the sum of CBDa and CBD. Although CBD comes from the decarboxylation of CBDa, both substances may have different effects in some respects. Therefore, the correct procedure would be that the product specify the content of CBD and CBDa separately and not as the sum of both.
Based on the values obtained by Fundación CANNA and considering the total CBD as the sum of CBD and its decarboxylated acid form, only in two products was it found that in both batches analysed the declared content was less than 10% of that found in the analysis.
5 of the products had a CBD content of less than 10% of that declared in the first batch analysed, however, the same five had correct values in the second batch. Only one product had lower values in lot 2 but correct in lot 1.
The causes of the discrepancies between the amount declared and that found in the analysis may be diverse. To name a few, special attention should be paid to the proper storage of these products by distributors, as factors such as temperature can lead to rapid degradation of cannabinoids in the oil. It is also highly recommended that producers carry out product stability studies in order to observe how the concentration varies over time.
In sanitary terms, the products were analysed to determine the content of heavy metals and pesticides present in them. Although heavy metals and/or pesticides have been found in some of the products, the quantities are so low that they do not pose a serious health risk.
It should be borne in mind that these products are not medicine and are not sold as such, so that medicinal quality criteria cannot be applied to them. The results of this study might serve as a basis to encourage manufacturers to pay more attention to aspects that permit the offer of a product that is more stable and in line with what the user expects.